The role of a battery in a circuit
The emf of a battery
A source of emf is a device that uses some form of energy (other than electrical energy) and coverts it to electrical potential energy. For example, in a battery, the chemical reactions taking place within the body of the battery creates a separation of charges at its two electrodes, so that one becomes positive and the other negative. This increases the electrical potential energy of the system of charges, and we can see that the battery does positive work in achieving this separation. The chemical energy is converted into electrical energy. In case of generators, which we shall see later in the course, mechanical energy is converted into electrical energy.
When the two ends of the battery are connected, the electrons from the negative end can move through the circuit to reach the other positive end of the battery. As they pass through the circuit components they lose part of their potential energy in each, so that when they reach the positive electrode, they have zero potential energy. The batteries role is to continuously keep supplying the electrons with a certain potential energy so that the flow of electrons is maintained in the circuit. The amount of electrical potential energy that the battery can provide to the electrons is clearly related to the potential gradient that is created as a result of charge separation, and hence the battery “value” is written in terms of the potential difference that exists between its electrodes. This is called the emf of the battery.
“The emf (ξ) of a device is defined as the work done by the source of emf in moving the charge around a closed loop or circuit.”
ξ = W/q (Units: J/C ≡ V)
Thus, a source of emf supplies electrical energy in a circuit, whereas the other components in the circuit (example resistors) dissipate that energy. Hence we can say that the emf supplies electrical Power, and a resistor dissipates electrical Power (in case of simple resistors, the electrical energy is converted into thermal energy).
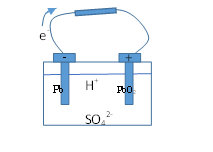
Let us look at a standard lead-acid battery:
Pb and plates are submerged in .
Pb + SO42- -> PbSO4 + 2e-
These electrons move in the wire and when they reach the positive plate:
PbO2 + 4H+ + SO42- + 2e- -> PbSO4 + 2H2O
Thus in this process PbSO4 is deposited on both plates and the acid is used up, and because of this there is a life span of the battery, after which no further reactions can take place since all the active ingredients have been used up.
(Rechargeable batteries are based on reactions that can be reversed by passing a current through them in the opposite direction with the help of another source of emf. So in this case, clearly the battery that is being charged is consuming Power that is being supplied by the other source of emf).